Science
THE CLINICAL RATIONALE
- Mitochondrial deficiency, characterised by a reduced number of mitochondria or impaired mitochondrial function, is a key factor in many health conditions.
- Mitochondria play a vital role in cellular energy metabolism, particularly in organs with high energy demands, such as the brain and heart.
- Recent studies support that poor mitochondrial health is closely associated with cognitive decline, such as Alzheimer’s and Parkinson´s diseases.
- Mitochondrial dysfunction is an attractive target for treatment regimes for neurodegenerative and metabolic diseases.
AI-based modelling of mitochondrial dysfunction
Mitochondria are energy factories of cells and are important pivots for intracellular interactions with other organelles in the human cells. Based on AI-based cellular modelling, it has been found that a mixture of metabolic activators can be used to restore mitochondrial function. These results open the door to entirely novel treatments for patients by restoring levels of Nicotinamide adenine dinucleotide (NAD+) and antioxidant providing entirely novel strategies for the treatment of the patients with mitochondrial dysfunction.
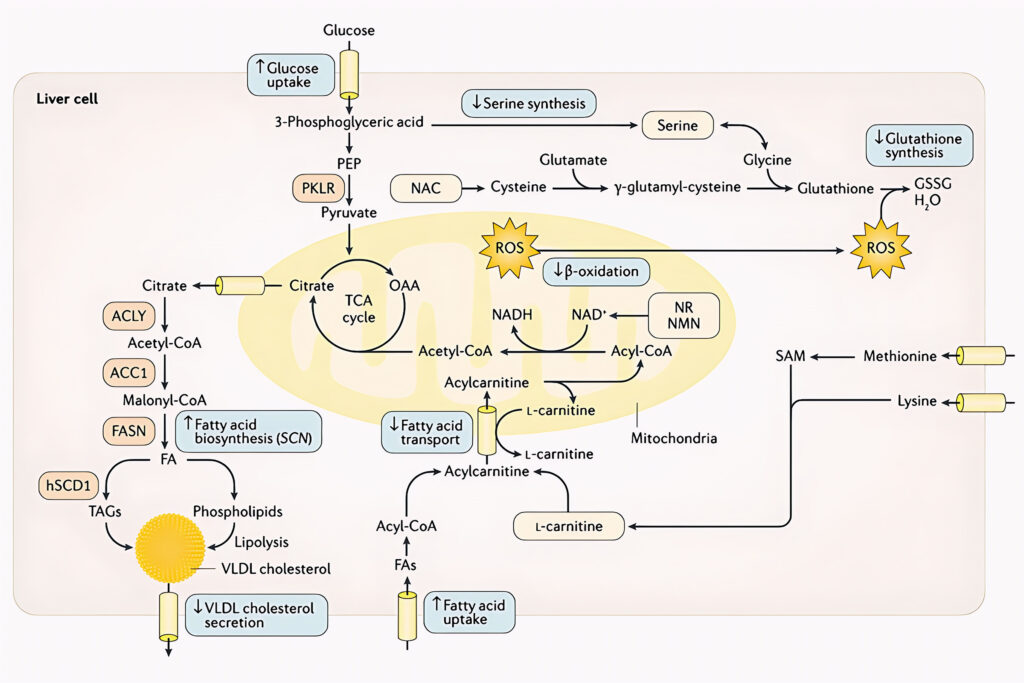
Well known and yet not considered until now
The scientific rational has been to employ a three-step strategy to activate the mitochondria in human cells:
L-carnitine tartrate is used to enhance the transport of fatty acids across the mitochondrial membrane (by forming a long chain acetylcarnitine ester to allow transfer of carnitine palmitoyltransferase [CPT] I and CPT II) and to stabilize acetyl-CoA and coenzyme A levels.
Nicotinamide riboside, precursor of NAD+ is used to boost the level of hepatic β-oxidation of fatty acids in mitochondria. Decreased electron transport chain function combined with impaired rates of fatty acid β-oxidation leads to the accumulation of incomplete products of β-oxidation, which combined with increased levels of reactive oxygen species (ROS). Nicotinamide riboside stimulates the transfer of fatty acids from cytosol to mitochondria, similar to L-carnitine tartrate.
Two glutathione precursors, serine and N-acetylcysteine, has been included to increase glutathione levels in the cells. Increased glutathione levels will also protect against free radical-mediated oxidative stress generated by the increased β-oxidation of fatty acids in mitochondria.
Cellular models, followed by animal studies and several human clinical trials have demonstrated the effectiveness of this three-step approach, targeting mitochondrial dysfunction.
AI-based drug development
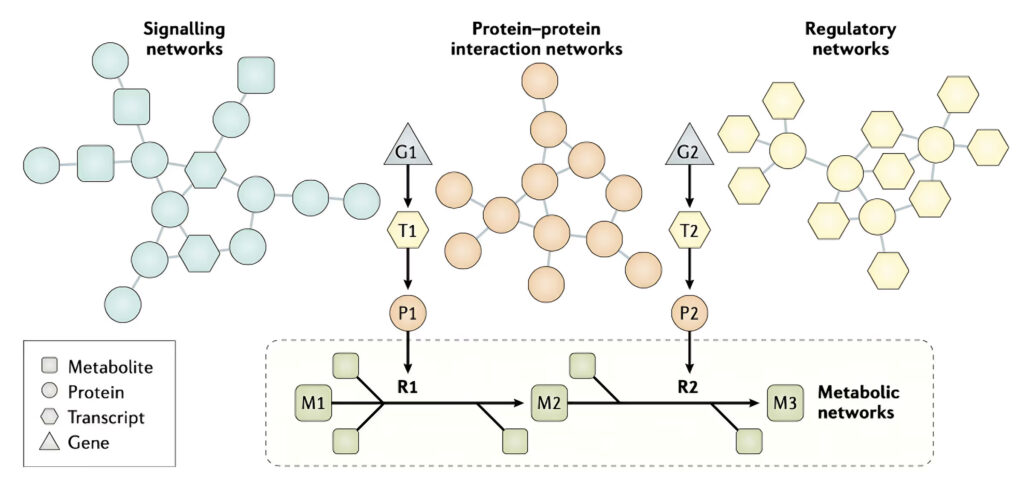
The latest algorithms and methods developed in AI has been used to integrate experimentally observed high throughput data, such as genomes, transcriptomes, proteomes, metabolomes and metagenomes for development of efficient treatment strategies for metabolism related diseases.
Individual biological networks, including metabolic, regulatory , signalling and protein–protein interaction networks, have been used in the analysis of omics data and to reveal the underlying molecular mechanisms of diseases to discover novel biomarkers and drug targets. High- quality omics data, including transcriptomics, proteomics, metabolomics and fluxomics, enable the generation of high- quality metabolic, signalling and protein–protein interaction networks. As shown in the figure, the effects of transcription factors and proteins on other proteins can be predicted by integration of different biological networks, and the level metabolites can be measured as a metabolic fingerprint in different clinical conditions. For example, gene 1 (G1) is transcribed to transcript 1 (T1), which is translated to protein 1 (P1); P1 then acts on metabolite 1 (M1) in reaction 1 (R1) to generate metabolite 2 (M2).
The early days…
In 2014, a liver- specific Genome-Scale Metabolic Model (GEM) was constructed following the generation of liver transcriptomics data, and this computer model was used to study the pathophysiological responses of hepatic disorders (1). This consensus functional GEM for hepatocytes, (iHepatocytes2322) was generated by integrating existing biochemical knowledge with the proteomics and transcriptomics data generated in the Human Protein Atlas (HPA) (2). The proteomics and transcriptomics data have been generated using the liver biopsy samples from MAFLD patients and healthy subjects. This computer model has been used to analyse the transcriptomics data generated for different stages of MAFLD using AI-based systems biology approaches.
In 2017, results from stable-isotope studies of triglyceride-rich VLDL cholesterol kinetics were combined with experimentally derived flux data to simulate liver metabolism using iHepatocytes2322 (4). The intracellular fluxes in the liver tissue of each of the patients involved in the study were predicted, and the correlations between the intracellular fluxes of the liver in patients with increased hepatic steatosis were assessed to elucidate the molecular derangements in the pathogenesis of NAFLD. The integrated systems approach indicated that altered NAD+ and glutathione metabolism (with an increased demand for NAD+ and glutathione) was a prevailing feature in MAFLD. Integrative analysis and plasma metabolomic data further indicated that plasma levels of serine, glycine and precursors of glycine are significantly negatively correlated with hepatic steatosis and suggested that serine/glycine is the limiting substrate for de novo glutathione synthesis.
To confirm these results and those of the earlier study indicating serine deficiency in patients with MAFLD, a short-term dietary supplementation study with one oral dose of ~20 g of L -serine (200 mg/kg body weight) per day was performed for 14 days in six patients with extensive hepatic steatosis (4). Serine supplementation was well tolerated by all the patients, and plasma levels of the liver enzymes alanine aminotransferase, aspartate transaminase and alkaline phosphatase — all well-known markers for liver health — significantly decreased after supplementation. Moreover, using magnetic resonance spectroscopy, it was found that hepatic steatosis significantly decreased after serine supplementation.
A system-level analysis suggested that a three-step strategy, including increased fatty acid uptake by mitochondria, fatty acid oxidation and generation of glutathione, might be advantageous for patients with MAFLD. Supplementation of the diet with natural substances including L -carnitine can activate the fatty acid uptake, precursors of NAD+, including nicotinamide or nicotinamide mononucleotide, can activate the fatty acid oxidation, and serine, glycine or N-acetylcysteine can activate the generation of glutathione, which might lead to a decrease in the level of the fat accumulated in the liver.
Human intervention studies
In 2018, we performed a short-term intervention with an isocaloric low-carbohydrate diet with increased protein content in obese subjects with MAFLD and characterized the resulting alterations in metabolism and the gut microbiota using a multi-omics approach (5). We observed rapid and dramatic reductions of liver fat and other cardiometabolic risk factors paralleled by (i) marked decreases in hepatic de novo lipogenesis; (ii) large increases in serum b-hydroxybutyrate concentrations, reflecting increased mitochondrial b-oxidation; and (iii) rapid increases in folate-producing bacteria and serum folate and serine concentrations. Liver transcriptomic analysis on biopsy samples from a second cohort revealed downregulation of the fatty acid synthesis pathway and upregulation of serine/folate-mediated one carbon metabolism and fatty acid oxidation pathways.
In 2019, we first assessed the tolerability in human subjects of the combined metabolic activators including L-serine, N-acetyl-L-cysteine (NAC), nicotinamide (NAD+), and L-carnitine by performing a 7-day rat toxicology study. We performed a human calibration study by supplementing combined metabolic activators (CMAs) and a control study to study the kinetics of these metabolites in the plasma of healthy subjects with and without supplementation. We measured clinical parameters and observed no side effects.
Next, we generated plasma metabolomics and inflammatory protein markers data to reveal the acute changes associated with the supplementation of the CMAs. We also integrated metabolomics data using personalized genome-scale metabolic modelling and observed that such supplementation significantly affects the global human lipid, amino acid, and antioxidant metabolism. Finally, we predicted blood concentrations of these compounds during daily long-term supplementation by generating an ordinary differential equation model and liver concentrations of serine by generating a pharmacokinetic model and finally adjusted the doses of individual metabolic activators for future human clinical trials.
Human phase 2 in fat liver patients
In 2020, we completed a phase 2 clinical trial testing the safety and efficacy of the combined metabolic activator (CMA) formulation in NAFLD patients in a placebo-controlled 10-week study (6). We found that CMAs significantly decreased hepatic steatosis and levels of aspartate aminotransferase, alanine aminotransferase, uric acid, and creatinine whereas found no differences on these variables in the placebo group after adjustment for weight loss. By integrating clinical data with plasma metabolomics and inflammatory proteomics as well as oral and gut metagenomics data, we revealed the underlying molecular mechanisms associated with the reduced hepatic fat and inflammation in NAFLD patients. In conclusion, we observed that CMAs could develop a pharmacological treatment strategy in NAFLD patients.
Human phase 3 clinical trials for COVID-19 patients
COVID-19 is associated with metabolic abnormalities, including the deficiencies in nicotinamide adenine dinucleotide (NAD+) and glutathione metabolism. In 2020, we also conducted randomised, placebo-controlled an open-label phase 2 study and a double-blinded phase 3 clinical trials for testing if a mixture of Combined Metabolic Activators (CMA) consisting of NAD+ and glutathione precursors had beneficial effects on ambulatory COVID-19 patients (7). 93 and 304 patients were assigned in phase 2 and phase 3, respectively, and administered CMA or placebo for 14 days. The clinical status daily was evaluated using a binomial scale for presence/absence for COVID-19 symptoms. The results of both studies showed that the time to complete recovery was significantly shorter in the CMA group (6.6 vs 9.3 days) in phase 2 and (5.7 vs 9.2 days) in phase 3 trials independent of standard therapy.
We unveiled the molecular changes by generating untargeted metabolomics and inflammatory proteomics data for all patients recruited in the phase 2 trial, showing that the plasma levels of proteins and metabolites associated with inflammation and antioxidant metabolism are significantly improved.
With hope for a better future
The scientific rationale – a summary
The aim of the novel drug treatment based on combined metabolic activators is to increase the level of NAD+ and glutathione in cells and tissues to boost the function of the mitochondria.
We have used natural substances, including serine, glycine and/or N- acetylcysteine (NAC) to increase the level of glutathione. In addition, precursors of NAD+, suitable for oral administration, such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), are added to the formulation to increase the level of NAD+ in cells and tissues.
Finally, the natural transport molecule L-carnitine is added to increase the transfer of fatty acids into the mitochondria of the cell. An effect of the activation of the mitochondria is also to decrease the level of the accumulated fat in the liver.
All of these natural substances can be administrated orally to promote mitochondrial fatty acid oxidation and thus potentially help patients with mitochondrial dysfunction.
References
- Yulug, B. et al. Combined metabolic activators improve cognitive functions in Alzheimer’s disease patients: a randomised, double-blinded, placebo-controlled phase-II trial. Transl. Neurodegener. 12, 4 (2023). doi:10.1186/s40035-023-00336-2
- Mardinoglu, A. et al. Genome- scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non- alcoholic fatty liver disease. Nat. Commun. 5, 3083 (2014).
- Uhlen, M. et al. Tissue- based map of the human proteome. Science 347, 1260419 (2015).
- Kalhan, S. C. et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 60, 404–413 (2011).
- Mardinoglu, A. et al. Personal model- assisted identification of NAD+ and glutathione metabolism as intervention target in NAFLD. Mol. Syst. Biol. 13, 916 (2017).
- Mardinoglu et al., An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans, Cell Metab. (2018),
- Zeybel et al., Combined Metabolic Cofactor Supplementation Reduces Liver Fat in Nonalcoholic Fatty Liver Disease, Under review at Cell Metabolism.
- Altay et al., Combined Metabolic Activators accelerates recovery in mild-to-moderate COVID-19, Under review at Advanced Science
